Autofluorescence
Autofluorescence is an important aspect of how cnidarians communicate with with reef coinhabitants. The specific patterns of autofluorescence function as a signalling mechanism to specific organisms capable of detecting the wavelengths emitted by cnidarians, much like how flowers use the UV spectrum to communicate with insects. (Gruber et al., 2008)
Specimens were collected from Frenchman’s Beach, Stradbroke Island. One specimen was selected to retrieve tentacles from for sectioning and staining. The specimen was relaxed using MgCl2 and a tentacle was then cut from the specimen using a scalpel. The tentacle was then placed in a tube and the seawater was removed. The specimen was then fixed in a 4% paraformaldehyde fixative in a MOPS fixation buffer (4% PFA) 100% ethanol. The tentacle was then cross-sectioned, mounted on slides and stained with phalloidin and DAPI (blue). Phalloidin binds to the actin filaments, therefore showing muscle fibres, and fluoresces at 600nm. DAPI (blue) fluoresces blue at 461nm and shows the nuclei of the cells. Pro-line gold was added to the top of the slide in order to attach the coverslips. The Nikon fluorescing compound scope was then used to examine the slides.
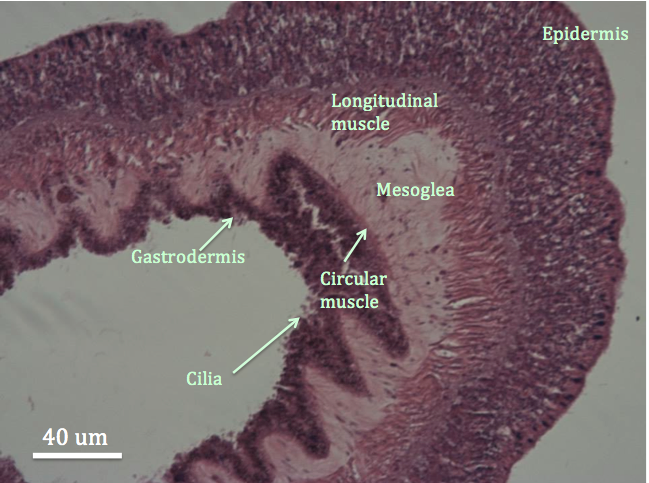
|
| Figure 1: Cross section of a tentacle of C. polypus stained with H&E. The hole in the middle of the tentacle is part of the gastrovascular cavity, which is surrounded by the gastrodermis. Important anatomical structures of the tentacle have been labelled. |
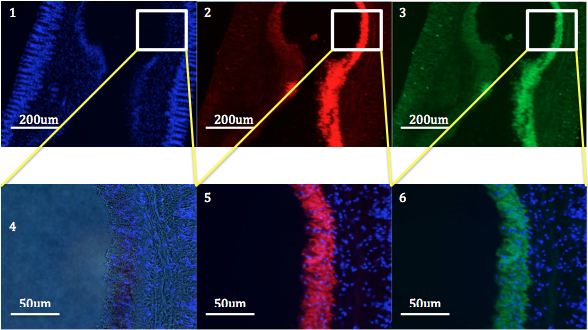
|
| Figure 2: Cross sections of a tentacle from C. polypus stained with fluorescent stains DAPI (blue) and phalloidin. Image 1 shows the fluorescent nuclei of the cells of the tentacle. Image 4 is a blown up section of image 1, to highlight the cells. Image 2 and 5 show red fluorescence, indicating autofluorescence. Image 3 and 6 show green fluorescence, indicating the presence of actin. |
Figure 2 shows the differential fluorescence produced within the tentacle of C. polypus. The layered images (5 and 6) show that the autofluorescence produced by the anemone and the fluorescence of the actin occur in the same region. Referring to figure 1, this region is the gastrodermal tissue and circular muscle.
Examination of the photos leans towards the hypothesis that it is possible that the patterns of fluorescence may not be related to the anemone’s appearance in natural light, but rather as a signalling mechanism to animals that are able to see the fluorescent wavelengths of light. One possibility for this hypothesis is that the fluorescence is associated with the feeding behaviour of the anemone. (Gruber et al., 2008) As flowers use the UV spectrum to communicate with, and attract insects and birds, the anemone uses the red fluorescence to attract prey, maximising its chances of intercepting prey items.
Another reason could be simply that the symbiotic zooxanthellae are autofluorescent. (Peng et al., 2010) Most Anthozoan species have a mutualistic symbiosis with photosynthetic zooxanthellae, which reside in the animal’s gastrodermal cells (Gruber et al., 2008). This suggests that the fluorescent patterning, as it is located within the gastrodermis, is not from the coral itself, but from the zooxanthellae living in the gastrodermal tissue. Both hypotheses cold be possible, however further investigation is needed.
|